Why Specific Heat Is Different for Different Substances Explain
Anabolic steroids also known more properly as anabolicandrogenic steroids AAS are steroidal androgens that include natural androgens like testosterone as well as synthetic androgens that are structurally related and have similar effects to testosterone. ə n ˌ h oʊ- species worldwide is about 1717 with the.
Thus air is a very good insulator of heat.

. The liquid used to prevent the different parts of a plant from getting too hot should have high specific heat. A word can have multiple and ambiguous meanings in everyday language but they have precise meanings in science. Which definition what one.
Write up your procedure. If substances A and B are liquids then which one would be more useful in car radiators. Carefully consider the order in which you will try each step.
Substances that do not conduct heat very welfare called bad conductors or poor conductors or insulators of heat. A note of caution. Design a procedure for separating the different substances in your mixture.
For example sugar or salt dissolved in water alcohol in water etc. Molar Specific Heat capacity is further divided into two types. Explain why a balloon would get bigger as it gains altitude.
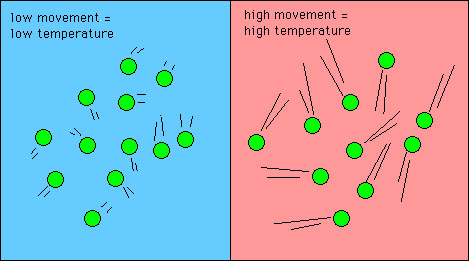
This equation is commonly used in order to. In general the specific heat also depends on the temperature. Shows molecules in two bodies at different temperatures and for hot and cold If two molecules collide energy transfers from the high-energy to the low-energy molecule.
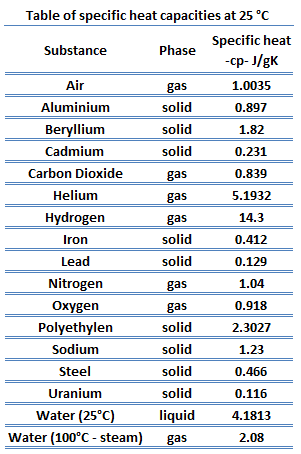
For example as you read above cardiovascular diseases have some related factors very specific to that issue. Values of specific heat must generally be looked up in tables because there is no simple way to calculate them. This allergic reaction can initiate an inflammatory process which can manifest topically or extend to other regions of the body.
Which of these do you want. Explain why a balloon would get bigger as it gets hotter. ə ˌ h oʊ-They are marine animals with a leathery skin and an elongated body containing a single branched gonadSea cucumbers are found on the sea floor worldwide.
Although many factors are broad enough to cut across many if not most community health and development concerns there are still some factors specific to different issues. Liquids like water are poor conductors and gases are very poor conductors of heat. PROCEDURE Examine the mixture and the materials provided.
Types of Molar Specific Heat Capacity. A heterogeneous mixture consists of visibly different substances or phases. Of 1 g of a substance 1C.
So less heat energy passes through the substance. Explain why a Two bodies at different temperatures T 1 and T 2 if brought in thermal contact do not necessarily settle to the mean temperature T 1 T 2 2 b The coolant in a chemical or a nuclear plant ie. Instead they are measured empirically.
Which do you want. In a metal the picture would also include free valence electrons colliding with each other and with atoms likewise. Reasons why an allergic reaction occurs including touching certain substances eating certain foods consuming other products or even inhalation of an allergen.
Some of the examples of heat or thermal insulators are plastic wood paper cloth thermocol rubber etc. The three phases or states of matter are gas liquid and solid. Separating each of these substances from the mixture.
5P31 Explain the effects of the transfer of heat either by direct contact or at a distance that occurs between objects at different. Specific heat capacity B 04 Jg K. Specific heat capacityA 38 Jg K.
Helpful specific and comprehensive for educators. They increase protein within cells especially in skeletal muscles and also have varying degrees of virilizing effects including. Efficiency in physics and often for chemistry is a comparison of the energy output to the energy input in a given systemIt is defined as the percentage ratio of the output energy to the input energy given by the equation.
FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. Which protects the surface of the shuttle from the heat of re-entry. Investigate and explain what the kinetic theory of gases states.
Explain the different diffusion speeds through substances in solid liquid and gas. The table below lists representative values of. 5L11 Explain why some organisms are capable of surviving as a single cell while others require many cells that are specialized to.
When the substances are heated we only consider the amount of energy to be added or removed and do not specify anything about the phase change. The number of holothurian ˌ h ɒ l ə ˈ θj ʊər i. A molecular picture of heat conduction will help justify the equation that describes it.
Since specific heat capacity is the energy required to raise the temp. It is because there is no change in temperature during the phase change. Explain the different properties of each group of materials.
Explain why you chose the steps you did for each substance. Sea cucumbers are echinoderms from the class Holothuroidea ˌ h ɒ l ə ˌ θj ʊəˈr ɔɪ d i.
What Is Heat Capacity Specific Heat Capacity Definition


Comments
Post a Comment